The bioresorbable Absorb scaffold considered the holy grail of coronary intervention is now being pulled out from the commercial market in Europe. The stunning news is the culmination of sticky data emerging from recent randomized trials assessing the bioresorbable vascular scaffold (BRS), that have revealed that with this generation of BRS both efficacy and safety are questionable.
The ABSORB II trial reported a significantly higher rate of target vessel myocardial infarction last year. The ABSORB III trial further dampened enthusiasm by showing significant increase in target lesion failure. The last randomized trial to be published was actually terminated early because of increased stent thrombosis including late stent (BRS) thrombosis.
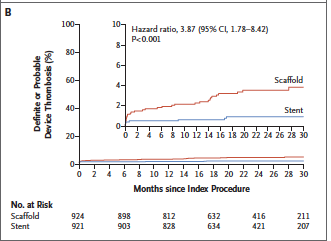
The AIDA (Amsterdam Investigator Initiated Absorb Strategy All Comers Trial) investigators randomly assigned 1845 patients to either receive a BRS (924 patients) or a metallic stent (921). Median follow up was 707 days. Target vessel failure (a composite of cardiac death, target vessel MI, or target vessel revascularization) occurred in 105 patients with BRS and in 94 patients in the stent group. Target vessel MI occurred in 48 patients in the BRS group but in only 30 provided metallic stents. Definite or probable stent thrombosis occurred in 31 patients in the scaffold group as compared with 8 patients in the stent group (3.5% vs. 0.9%; p<0.001). This was a single blind, multicenter, investigator initiated non-inferiority trial.
AIDA has some limitations. Predilation with a 1:1 balloon to artery ratio with an appropriately sized balloon was not performed in all patients. Intra-vascular imaging during BRS implantation too was not performed in all patients, and a median 2-year follow up would be considered too short.
This moreover, is a preliminary analysis from the trial because of premature termination due to safety concerns. The increased device thrombosis seen in the IADA trial has been reported earlier in 2 randomized trials and in several meta-analyses.
A meta analysis of 4 randomized trials showed a higher rate of device thrombosis than with metallic stents (relative risk was 2.09; p=0.08). Target vessel myocardial infarction too was raised significantly in the scaffold device group.
A systemic review of 6 randomized trials and 38 observational studies has also shown higher rates of device thrombosis than with metallic stents (odds ratio for device thrombosis 2.32; p=0.04) and myocardial infarction (odds ration 2.09; p= 0.007). Hence the need for more contrast use, greater fluoroscopy time during device deployment, with need for more hardware for the procedure add to disadvantages of BRS deployment against the background of significantly greater thrombosis. The vessel has to be predilated with a non compliant balloon (1:1 balloon –artery ratio), sizing has to be confirmed by IVUS or optical coherenece tomography (OTC) or quantitative coronary angiography and post dilation up to 0.5 mm larger than the nominal scaffold diameter is considered mandatory.
Prolonged dual anti-platelet therapy (DAPT) is recommended with BRS, and was indeed recommended by the safety board of AIDA. Extended duration of DAPT invariably comes with the price of greater bleeding.
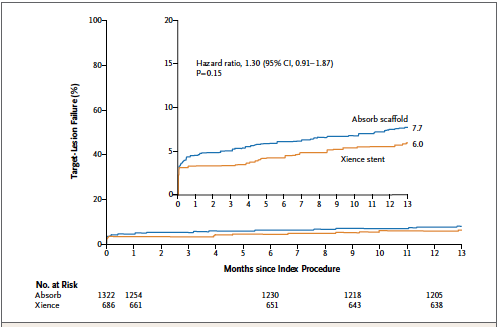
The largest trial with the bioresorbable scaffold, the ABSORB III trial (included 2008 patients) reported non inferiority when compared to the Xience everolimus stent with regard to cardiac death, target vessel myocardial infarction or ischemia driven target lesion revascularization at 1 year (rate of 7.8% with the scaffold vs. 6.1% with the Xience stent. The rate of device thrombosis by 1 year was higher in the scaffold group than in the metallic stent group (1.5% vs. 0.7%, p=0.13). Device thrombosis albeit not increased significantly was more than twice with the scaffold. By 2 years the rate of target lesion failure had become significantly higher in the bioresorbable scaffold group than in the metallic stent group (11% vs. 7.9%; p=0.03).
Drug eluting stents (DES) have for long become standard treatment in acute coronary syndrome. But DES are hampered by neoatherosclerosis that can cause late stent thrombosis (rate of 0.1% to 0.2% per year), there can be repeat revasularization (rate 2% to 3% per year), and because they are rigid metallic tubes they compromise vasomotion. These are the primary reasons for development of the bioresorbable scaffold. But for now the first generation scaffold have failed to live up to their promise both for safety and efficacy.
The causes of higher rate of device thrombosis could be the large size of the struts (150 microns) that are associated with associated with blood flow alterations and thrombogenecity. Incomplete lesion coverage, malapposition, or under deployment also play a role in greater thrombogenecity. Hence newer generation devices with thinner struts, greater radial strength and quicker dissolution could cover up the inadequacies of the current bioresorbable scaffold.
The company has rightly withdrawn the device from the commercial market in order to sort out the problem of increased thrombosis. The device will be continuing to be used in ongoing registries. Strangely the Absorb device continues to be marketed in the USA and also India. Ironically almost the entire interventional cardiology world had been raving about the bioresorbable scaffold for the last 5 years. It is now back to the drawing board to fix the safety issues, and the answers will take a long time coming out. Till then the best bet should be the newer drug eluting metallic stents for routine percutaneous intervention and treatment of acute coronary syndromes.