Dabigatran a direct thrombin inhibitor came into reckoning with the RELY trial published in the New England Journal of Medicine in 2009. More than 18000 patients of atrial fibrillation with increased risk for stroke were randomized to receive 110 mg or 150 mg of dabigatran twice a day in blinded fashion compared with warfarin adjusted to therapeutic INR in the remaining cohort. Warfarin, a vitamin K antagonist, has been used as an effective anticoagulant for decades. Warfarin reduces the risks of stroke by two thirds in patients with atrial fibrillation. Atrial fibrillation is the commonest sustained arrhythmia in the world, leading to stroke, systemic embolism, heart failure, ventricular arrhythmia and death. Warfarin however has been a hard task master, it has a bad efficacy/ risk ratio, tricky interactions with drugs and food, wide ranging blood level necessitating frequent blood monitoring. Apart from dabigatran the other non vitamin K antagonist oral anticoagulants (NOAC’s) available in the market are the direct factor Xa inhibitors, apixabn, rivaroxaban and edoxaban.
Dabigatran was launched with the promise that it did not have the awkward baggage that accompanied warfarin. It had a small half life of 12-18 hours, reacted little with food or other drugs, and above all did not need any monitoring. The primary outcome of stroke and systemic embolism was, at the end of 2 years, 1.7% with warfarin, 1.5% with 110 mg dabigatran and 1% with 150 mg of dabigatran. Major bleeds were 3.7% with warfarin, 3.1% with 150 mg dabigatran and 2.7% with 110 mg dabigatran. Haemorrhagic stroke were 0.4% with warfarin, and about 0.1% with both doses of dabigatran( significantly less). Mortality was 4.3% with warfarin, and around 3.7% with both dabigatran groups. In patients with atrial fibrillation, dabigatran at 110 mg twice a day had similar rates of stroke and systemic embolism but lower risk of major haemorrhage. On the other hand the 150 mg twice a dose of dabigatran had significantly fewer stroke and systemic embolism but at equivalent rate of major haemorrhage.
To prevent one non haemorrhagic stroke around 357 patients will be needed to be treated with 150 mg dabigatran twice a day as compared to warfarin. Also to prevent a haemorrhagic stroke 370 patients would need to be treated with dabigatran as compared to warfarin for a period of 2 years, because the rate of haemorrhagic stroke were about 0.1% in both dabigatran as compared to warfarin with a rate of 0.4%, an absolute difference of 0.3%. Warfarin was maintained in the therapeutic range in about two thirds of patients.
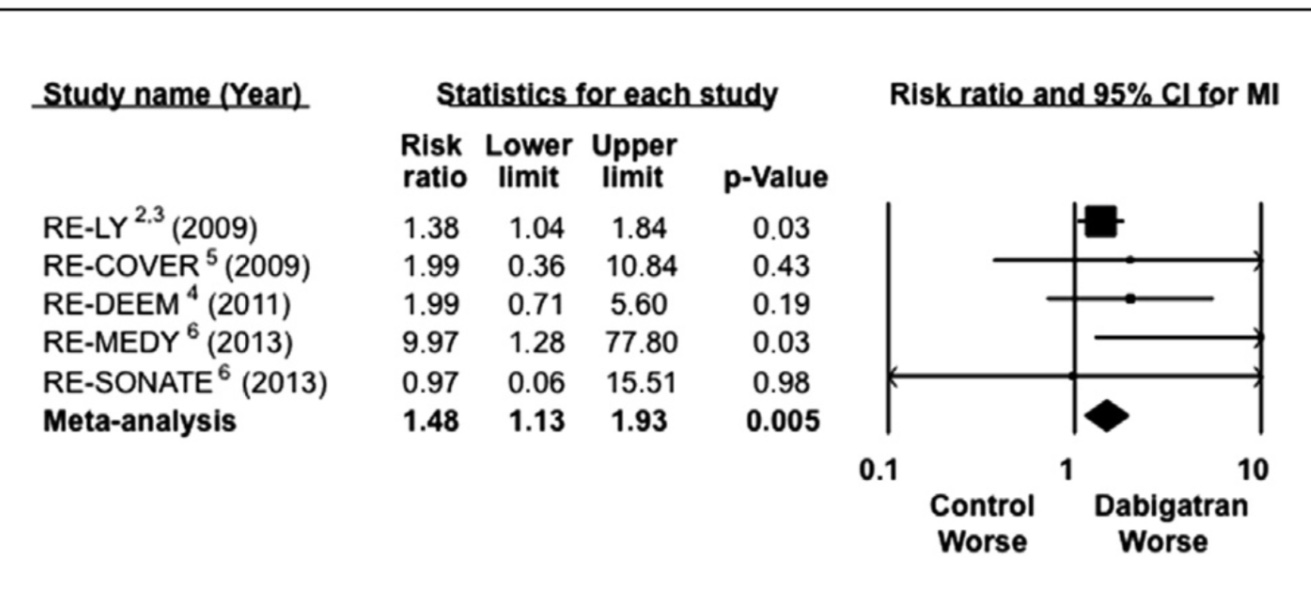
Dabigatran however produced more myocardial infarction and gastrointestinal bleeds. The rate of myocardial infarction was 0.5% with warfarin whereas it was about 0.7% in both dabigatran groups. The mechanism of increased rate of myocardial infarction with a thrombin inhibitor is unclear at present. Another anti thrombin agent ximelagatran has also been associated with increased rate of myocardial infarction.
Against this background of the possibility of increased risk of myocardial infarction the latest big study with dabigatran called the MANAGE trial is bound to draw a lot of attention. The MANAGE trial presented at the ACC 2018 meeting has come up with a new disease entity called myocardial ischemia after non cardiac surgery or MINS. MINS is diagnosed by raised troponin levels and almost 90% of patients in MANAGE were asymptotic. The patients who had suffered a myocardial insult were randomized to 110 mg dabigatran twice a day versus placebo. The primary outcome was a composite of death, myocardial infarction, ischemic stroke, peripheral artery disease, amputation, and acute venous thromboembolism. Dabigatran lowered ischemic outcomes by an absolute 4% as compared with placebo, from 15% to 11%. Hence only 25 patients will be needed to be treated to prevent these ischemic clinical events. Surprisingly there was no difference in bleeds in the 2 groups. Moreover almost half the patients stopped taking dabigatran.
In MANAGE, 80% of patients with MINS were diagnosed on the basis of increased troponin values, while 20% of patients had myocardial infarction based on standard definition. The trial included more than 1700 patients with a diagnosis of MINS at an average of 5 days post non cardiac surgery. Almost 50% were women. About two thirds of patients were on aspirin and a statin. Duration of follow up was 16 months, with mean patient age being 70 years.
The questions that will be raised are whether all patients undergoing non cardiac surgery have a troponin check? In the event they are raised should these patients be administered an anticoagulant along with aspirin? For how long? We will need to wait for more randomised trials that answer these queries. The fact that only 50% of patients got to finally included in the trial with almost half of dabigatran patients decided to stop taking the drug makes the study a trifle unconvincing. But a myocardial infarction following surgery can have a grim prognosis. These are not typical due to a ruptured atherosclerotic plaque in the coronary artery but because of an oxygen supply demand mismatch, which can be due to a fast heart rate, fever, hypotension or hypovolemia accompanying non cardiac surgery. Such a myocardial infarction is termed type 2 as per universal definition of acute myocardial infarction. The MANAGE trial may compel physicians not only to measure troponin levels post non cardiac surgery but also contemplate whether to administer dabigatran in patients with MINS. It should be also noted that the trial was terminated early, and the primary outcome definition was changed midway.
A large retrospective study published last year in the Annals of Internal Medicine compared 25000 patients of non valvular atrial fibrillation on dabigatran to 25000 patients on warfarin in a propensity score matching system. This large study concluded that ischemic stroke were similar in both groups but haemorrhagic stroke were less with dabigatran (0.8% versus 0.4%). Rates of myocardial infarction were again found to be more with dabigatran than with warfarin (0.8% versus 0.4%) . Older people and those with kidney disease had greater GI bleeds with dabigatran. The researchers cautioned that association between myocardial infarction and dabigatran remained unclear, but warrants further investigation.
Observational studies assessing “real world” patients have concluded that there is no increase of myocardial infarction with dabigatran, but randomised trials have repeated the concern raised by the RELY study. Several randomised trials have shown increased myocardial infarction with dabigatran. In fact a combined analysis reveals a 48% excess in myocardial infarction with dabigatran as compared to placebo. The PETRO trial hypothesised that in the absence of aspirin dabigatran increases urinary secretion of thromboxane metabolite, indicating heightened platelet activity. Increased platelet activity when dabigatran is used without concomitant may explain increased myocardial infarction.

However there was no increase in composite of ischemic events (death, myocardial infarction, sroke) with dabigatran combined with clopidogrel or ticagrelor compared to warfarin triple therapy after PCI in patients with atrial fibrillation.The RE Dual PCI study randomly assigned 2725 patients with atrial fibrillation who had undergone PCI to triple therapy with warfarin plus clopidogrel or ticagrelor and aspirin , or dual therapy with dabigatran plus clopidogrel or ticagrelor. He primary endpoint was a major bleed. Major bleed ing was lowered from 27% in the warfarin triple group to only 15% in 110 mg dabigatran group, and from 27% in the warfarin group to 20% in 150 mg dabigatran group. The CHA2DS2-VASC score was 3.7 in all 3 groups. The HAS BLED score was around 2.7. Almost 25% of patients had history of previous myocardial infarction. Myocardial infarction increased from 3% (warfarin triple therapy) to 4.5% (110 mg dabigatran) , and from 2.9% to 3.4% in the 150 mg dabigatran group. The increase in MI was not significant.
One big advantage with dabigatran is that we have an effective antidote. A monoclonal antibody called idarucizumab (which is available in India) was approved in October 2015 by the US FDA. Dabigatran inhibits thrombin which is one of the later stages in the coagulation cascade. Idarucizumab reverses dabigatran’s anticoagulant effect by tightly binding to dabigatran with an affinity 350 timers greater than thrombin. Studies have shown that idarucizumab almost completely reversed dabigatran’s anticoagulant effect in healthy volunteers aged 65-80 and volunteers 45-80 with mild to moderate renal dysfunction. The reversal lasts for 24 hours. Idarucizumab is given as 2 boluses of 2.5 grams each, within 15 minutes of each other. The 2.5 gm bolus is given over 5 minutes. Adverse events with Idarucizumab are rare and include hypkalemia and headache.
A total of 503 patients with uncontrolled bleeding (group A) and need for emergency surgery (group B) were included in a multicentre trial of idarucizumb. Gastrointestinal bleeding was present in 45% and intracranial haemorrhage in 33% patients in group A. Idarucizumab rapidly and safely reversed the anticoagulant effect of dabigatran in almost all patients (NEJM; 377:431).
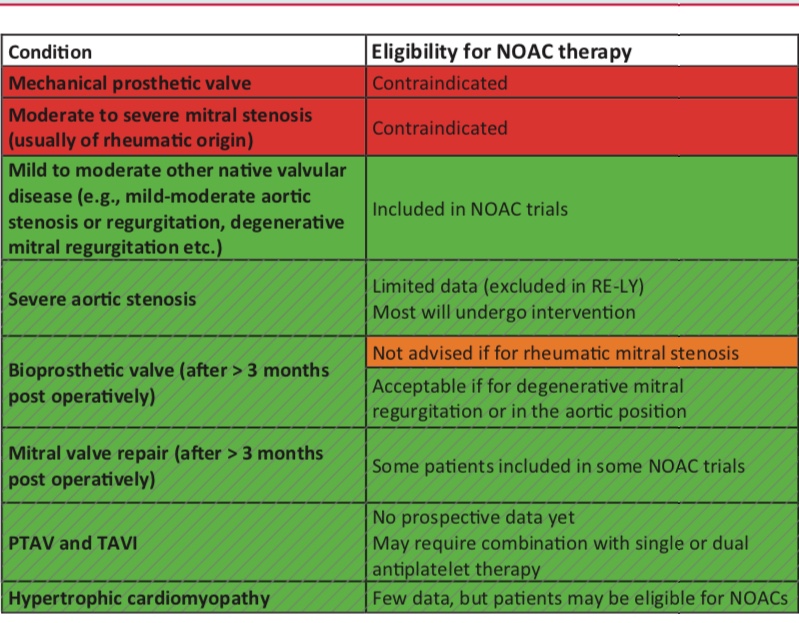 The take home message is that in the RELY study, dabigatran 150 mg twice a day reduced stroke and systemic embolism by 35% compared to warfarin without a significant difference in bleeds. Dabigatran 110 mg twice day was non inferior to warfarin for prevention of stroke and systemic embolism, but resulted in 20% lesser major bleed. Both doses of dabigatran significantly reduced incidence of intracranial haemorrhage. There was a non significant increase in rate of myocardial infarction in both doses. A bleeding emergency with Dabigatran can be effectively treated with 5 gm of IV Idarucizumab. Dabigatran should be used with caution or not at all in severe kidney dysfunction (Cr Cl< 30 /m). There is no randomised data on Dabigatran in patients on dialysis. Dabigatran cannot be used in patients with mechanical valves or in patients with mitral stenosis. The bleeding advantage of 110 mg Dabigatran over warfarin is lost in patients with CrCl <50 L/m while maintaining a similar stroke risk reduction. The 110 mg twice a day dabigatran dose is recommended with Cr Cl < 50 L/m. A dose of 75 mg twice a day of Dabigatran in CrCl < 30 L/m has been approved in the US. Dabigatran is contraindicated in patients with liver disease associated with coagulopathy and clinically relevant bleeding risk.
The take home message is that in the RELY study, dabigatran 150 mg twice a day reduced stroke and systemic embolism by 35% compared to warfarin without a significant difference in bleeds. Dabigatran 110 mg twice day was non inferior to warfarin for prevention of stroke and systemic embolism, but resulted in 20% lesser major bleed. Both doses of dabigatran significantly reduced incidence of intracranial haemorrhage. There was a non significant increase in rate of myocardial infarction in both doses. A bleeding emergency with Dabigatran can be effectively treated with 5 gm of IV Idarucizumab. Dabigatran should be used with caution or not at all in severe kidney dysfunction (Cr Cl< 30 /m). There is no randomised data on Dabigatran in patients on dialysis. Dabigatran cannot be used in patients with mechanical valves or in patients with mitral stenosis. The bleeding advantage of 110 mg Dabigatran over warfarin is lost in patients with CrCl <50 L/m while maintaining a similar stroke risk reduction. The 110 mg twice a day dabigatran dose is recommended with Cr Cl < 50 L/m. A dose of 75 mg twice a day of Dabigatran in CrCl < 30 L/m has been approved in the US. Dabigatran is contraindicated in patients with liver disease associated with coagulopathy and clinically relevant bleeding risk.