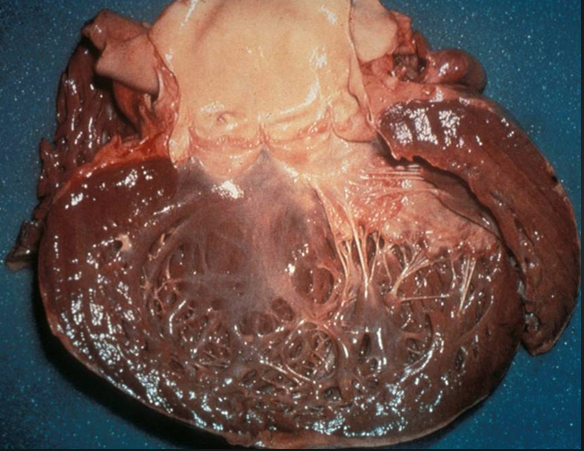
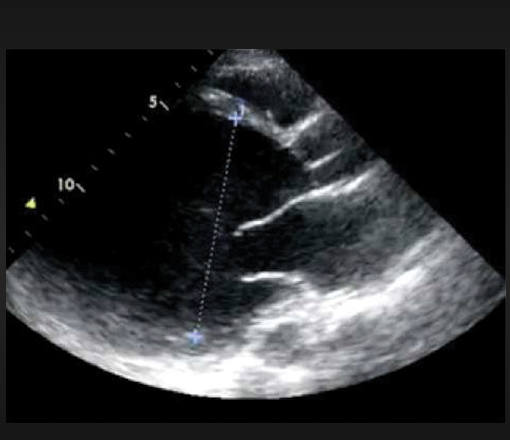
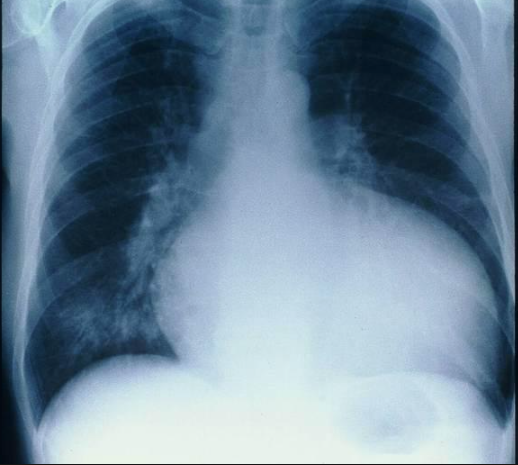
Peripartum cardiomyopathy (PCM) is an uncommon disease in which pregnant women present with systolic heart failure (during last month of pregnancy and with 5 months post of delivery of the baby). The incidence of PCM varies from 1 in 100 in Haiti, 1 in 300 in Nigeria and 1 in 4000 in Europe. Advanced age, preeclampsia and twins are well known risk factors for PCM. The disease may not resolve in a sizeable number of patients and carries a death rate of at least 10%. One fourth of deaths are attributed to sudden cardiac death because of ventricular tachyarrhythmias.
The cause of PCM remains unknown. Various hypotheses have been presented such as selenium deficiency, fetal autoimmunity, myocarditis and excess salt intake. There is however still no clear explanation as to why heart failure develops in a small number of pregnant women. Genetic factors have always been suspected especially because of higher prevalence in hot spots such as Haiti and Nigeria. Familial clustering of PC has been noted with 15% of patients in a study of German women had history of PC, dilated cardiomyopathy (DCM) or sudden death in first degree relatives.
New research published on line in the NEJM has noted that mutations in the TTN gene commonly found in DCM are also found in a considerable number of patients with PCM. The study has shown that 15% of 172 women with PC had similar variations in 8 genes as found in 17% of patients of 332 patients with DCM. The majority (65%) of these genetic variations occurred in the TTN gene suggesting that these genetic variations made women susceptible to development of PCM. It thus appears that both diseases have a shared mechanism and this would aid in future remedies.
The TTN gene encodes the sarcomere protein titin, which is a component of cardiac muscle. Almost 25% of patients with familial DCM and 18% with sporadic DCM have truncated TTN. The researchers sequenced DNA from 172 patients with PCM to check for genetic abnormalities similar to patients with DCM and discovered that most had variation in TTN.
Women with gestational hypertension who developed PCM did not carry TTN abnormalities, whereas women who had genetic variation did not suffer from hypertension, suggesting different pathogenesis. Interestingly the authors also recorded that onset of DCM I women with TTN abnormality was around 65 years while onset was 28 years in case of PCM. This the researchers discuss implies that environmental factors must be at play also. Greater recovery is seen with PCM than in patients with DCM but for the first time we now know that certain subset of women are predisposed to development of systolic heart failure due to alterations in the TTN gene.
The study found 26 truncating variants in 8 genes in women with PCM; the prevalence of truncating variants was significantly higher than a control population of 60,706 persons. (15% vs. 4.7%), but was similar to that found in patients with DCM (55 of 332 patients or 17%) Two thirds of these truncated variants were seen in TTN.
For the present, women with PCM can be divided into 2 groups; with TTN variation or without; more research are needed to explore the disease in greater detail in order to provide tailored treatment. We are now armed with the knowledge that PCM in some women can have a genetic cause.
An interesting article published in 2014 in jACC studied (retrospectively) the improvement in left ventricle ejection fraction (LVEF) in 100 patients with PCM. There were 55% African women, 39% Caucasians and 6% Hispanics in this cohort, with mean age of 30 years and mean LVEF was 28%. Improvement in LVEF was documented in 42% of patients over follow up of approximately 3 years. Seven of 58 (12%) patients who did not have improvement in LVEF underwent implantation of an implantable cardioverter-defibrillator (ICD). There were 11 deaths. Recovery was greater in caucasians and in those discovered to have PCM postmortem. Treatment was similar in patients who died and those who survived and this included beta-blockers and angiotensin converting enzyme inhibitors/angiotensin receptor blockers. Base line LVEF was not found to be a predictor of recovery; and the authors recommend that an ICD may be implanted in patients with PCM who fail to improve over 6 months. A life vest could be considered in the interim period if the patient displayed frequent ventricular ectopy.
We also have information now on the fate of subsequent pregnancies (SP) in patients with PCM. The 2 largest studies with a total of 105 SP’s demonstrated relapse with worsening of symptoms and lowering of LVEF in almost one-third of cases. The risk of relapse in persistent LV dysfunction is significantly greater than in patients with normalised left ventricle function; and this risk is as much as 50% with little chance of recovery after delivery. Symptomatic deterioration during pregnancy in patients with persistent left ventricular dysfunction could also be due to increased haemodynamic burden of pregnancy. Twenty percent of patients with recovery of left ventricle function will develop deterioration during subsequent pregnancy which persists after delivery in 20% to 50% of patients.
Preliminary research suggests potential efficacy of bromocriptine in women with SP’s. A small study compared 6 patients with PCM and SP who reviewed bromocriptine upto 3 months post delivery and standard therapy with women who did not. All 6 women on bromocriptine survived and had preserved left ventricle ejection fraction. In the 6 patients who did not receive bromocriptine all had deterioration in left ventricle function and 3 women died in 4 months. All patients in this study began with similar left ventricle function.More data is needed to establish the role of bromocritine in PCM.
Another small prospective randomised study involving 20 women with PCM , administered bromocriptine 2.5 mg twice daily for 2 weeks and then 2.5 mg daily for 6 weeks , in addition to standard heart failure therapy. The authors concluded that bromocriptine improved left ventricle function and clinical outcomes albeit the number of patients were few and clinical results could therefore not be considered definitive. Bromocriptine suppresses lactation and there are reports of myocardial infarction, stroke and seizures.
Clinically stable women with PCM should not be discouraged from breast feeding because negligible amounts of standard heart failure drugs are concentrated in human milk. The amount of captopril or enalapril concentrated in human milk is insignificant. No reports are available on beta blockers such as carvedilol and metoprolol succinate during human lactation but the amount of metoprolol present in human milk is very small. It is advised that breast feeding is done 3-4 hours after the last dose of metoprolol to further reduce exposure. Similarly, the active metabolite of spironolactone, candrenone is found in insignificant amounts in human milk. Spironolactone can therefore be safely administered in women with peripartum cardiomyopathy. There are concerns that bromocriptine by suppressing lactation may induce infection and malnutrition in infants in developing countries. It is therefore mandatory that large scale randomised trials are conducted with bromocriptine to establish its role in treatment of peripartum cardiomyopathy.
.