Heart failure (HF) is an awful disease, there are lakhs of patients in this country who barley get treated. A considerable proportion of HF patients have systolic dysfunction or reduced ejection fraction of there left ventricle. Thus is also known as heart failure with reduced ejection fraction or HFrEF. The burden of severe heart failure continues to escalate . Just about every practicing physician regularly comes across a heart failure patient needing careful treatment and probable admission in hospital. Once hospitalised almost 30% to 40% patients need re-hospitalisation within 6 months. Almost patients are dead due to worsening heart failure or sudden arrhythmia. The majority of current physicians will just not be able to appreciate that as recently as the mid ninety eighties there was no effective treatment for heart failure apart from diuretics and digoxin. Neither diuretics or digoxin are known to reduce death in HF. Despite reduction in hospitalisation by angiotensin converting enzyme (ACE) inhibitors, spironolactone and digoxin, the incidence of admission for worsening HF remains quite grim.
The 1990’s witnessed substantial progress in reduction of HF hospital admission. Beta blocker treatment in addition to ACE inhibitors and digoxin reduced risk of hospitalisation by 20% to 30%. The early studies on beta blockers assessed efficacy in patients with mild to moderate HF. The effects of beta blockers on morbidity in patients with severe HF were yet to follow. The COPERNICUS ( N Engl J Med 2001;344:1651) trial was one such pioneering trial that established the role of carvedilol in patients with severe HF. COPERNICUS was a prospective double blind placebo controlled trial that studied the effect of carvedilol on mortality and morbidity in severe HF. Severe HF was defined as breathlessness or fatigue at rest or on minimal exertion, and a left ventricle ejection fraction less than 25%. Patients were already on diuretics (>95%), an ACE inhibitor (>95%), digoxin (65%), spironolactone (20%) or amiodarone (15%). The trial was stopped early after a mean duration of follow up of 10.4 months, because of a highly significant benefit on survival with carvedilol. Notably in this short follow up 24% of patients in there placebo group were admitted for worsening HF as compared to 17% in the carvedilol group, indicating the severity of HF in this trial. Moreover there was no incidence of worsening of HF by carvedilol during start of treatment.
In the Discussion section the authors noted that for every 100 patients of severe HF treated for one year, carvedilol prevented 7 deaths. Other beta blockers or an ACE inhibitor prevent about 2-4 deaths in 100 patients of mild to moderate HF if treated for a year. Spironolactone could prevent 5 deaths if given for a year in severe HF patients. Carvedilol is different from other beta blockers in that it is a non selective adrenergic blocker. Unlike metoprolol that blocks only beta 1 receptor, carvedilol blocks both beta 1 and beta 2 receptors, it also blocks alpha 1 receptor. Beyond almost complete adrenergic blockade, carvedilol is an anti-oxidant and a vasodilator.
Chronic beta 1 adrenergic receptor signalling is the dominant cardiotoxic pathway in the failing heart. Second generation beta 1 selective blockers (metoprolol and bisoprolol) have been shown to reduce death and hospitalisation in HF. But as mentioned earlier beneficial effects of metoprolol and bisoprolol have been reported in moderate HF patients only. A post hoc sub group analysis of metoprolol succinate including about 800 patients only (MERIT-HF) ( J Am Coll Cardiol 2001; 38:932) showed that metoprolol succinate works effectively in severe HF patients also. The mean action fraction at baseline was 19%, and the yearly mortality during follow up was 19%. The subgroup analysis of the mERIT-HF study showed that patients with severe HF got similar mortality benefit and a similar reduction in hospitalisation with metoprolol succinate as those with moderate HF.
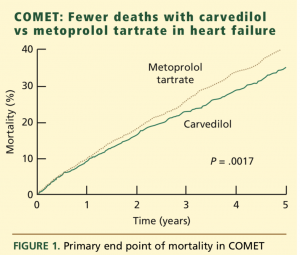
A direct head to head comparison of 2 different beta blockers was finally done. The COMET trial compared carvedilol (50 mg a day maximum dose) with metoprolol tartrate (maximum dose of 100 mg a day) in more than 3000 patients of moderate or severe HF. The COMET trial was conducted against the background that all beta blockers are not necessarily equal. Carvedilol was begun at 3.125 mg and titrated to the target dose of 25 mg twice daily, while metoprolol tartrate was started at 5 mg and titrated to the target dose of 50 mg twice daily. The primary end point was all cause mortality. Over a mean follow up of almost 5 years, 34% of patients on carvedilol died as compared to 40% in the metoprolol group (p=0.002). Difference in mortality first appeared at 6 months and the difference in mortality was not influenced by baseline characteristics. The combined end point of death or hospital admission did not however differ (about 76% in both groups). Carvedilol was noted to have significantly lower rates of death from stroke and new onset diabetes.
The mean dose of carvedilol was 42 mg/day and the mean dose of metoprolol tartrate was 85 mg/day. Metoprolol tartrate is an immediate release tablet and hence when given twice a day may provide ineffective blood levels. The MERIT-HF researchers have argued that the correct effective dose should have been 50 mg four times a day with metoprolol tartrate instead of 50 mg twice a day as used in the COMET trial. The basal heart rate however was 81/min in both groups and by 16 months heart rate was similar in both groups, suggesting that there was equivalence in beta blockade. But a better measure of beta blockade is heart rate on exercise, and this was not measured by the COMET investigators. The COMET trial (Lancet 2003; 362:7-13) indicates that carvedilol at a target dose of 50 mg/day is superior to metoprolol tartrate at a target dose of 100 mg /day in reducing all cause mortality in patients with chronic HF already on ACE inhibitor therapy.
Carvedilol appears to be an excellent drug to use in moderate and severe HF. Stable patients already on metoprolol succinate or bisoprolol should not be switched to carvedilol. A recent retrospective study( American Journal of Kidney Disease ;in press) in 27,000 patients on dialysis showed carvedilol resulted in greater all cause mortality at 1 year follow-up as compared to metoprolol, probably due to increased occurrence of intradialytic hypotension after carvedilol. Similar associations were observed in patients with hypertension, heart failure, atrial fibrillation and recent myocardial infarction. It would be worthwhile to take note of a trial published in the current issue of JACC ( J Am Coll Cardiol May 2018; 71: Issue 20 ), that enrolled 200 patients with breast cancer and normal left ventricle section fraction and randomly assigned them to placebo or carvedilol (maximal tolerated dose of 18.5 mg /day) at the start of anthracycline chemotherapy (cumulative dose, 240 mg/m2). Echocardiography was employed to assess ejection fraction at base line and after each chemo cycle or 6 months. At 6 months the primary endpoint of at least 10% decrease in ejection fraction occurred in 15% of carvedilol group versus 14% given placebo. There was thus no difference in change ion. Ejection faction with carvedilol. Carvedilol however significantly reduced levels of troponin I and percentage of patients with diastolic dysfunction, and was associated with trend to less decreased LV end diastolic diameter. The discrepancy in troponin rise without change in action fraction could be explained by the fact that the troponin rise may not be enough to affect remodelling of the left ventricle. Maybe a longer follow up would pick up an effect upon ejection fraction. The researchers concluded that albeit carvedilol had no impact on the incidence of early onset left ventricle ejection fraction reduction, but did result in significant reduction in troponin levels and diastolic dysfunction in patients of breast cancer (HER2 negative) administered contemporary anthracycline dosage treatment. Anthracyline is a widely used drug for chemotherapy in women with breast cancer. In non randomised randomised trials early administration of carvedilol and ramipril has shown promise in patients presenting with HF after anghrcaycline chemotherapy. The use of beta blockers for primary prevention of HF subsequent to chemotherapy continues to be controversial. Metoprolol succinate in the PRADA trial failed to prevent anthracyline cardiotoxicity ( European Heart Journal 2016;37:1671). Metoprolol dis have significant effect in cutting down rate of diastolic dysfunction.It is well known that some patients can withstand high doses of anthracycline without developing HF, whereas other patients land up with heart failure with a relatively small dose, suggesting considerable individual variabilty. In PRADA, candesartan reduced drop in ejection fraction as compared to placebo. But candesratan did not have any effect on left ventricle diastolic dysfunction, or level of troponin.
More randomised trials with longer follow up to recognise effective treatment for these patients.are needed to assess the efficacy of primary prevention in the setting of chemotherapy with anthracyline. Anthracycline induced heart failure is a terrible problem that can occur as late as 20 years after index chemotherapy. Cancer drug associated HF is a major health concern and it is imperative that we better understand it’s pathophysiology. Great cancer is after all there most common cancer in women in the developed world with HF being the most severe adverse effect of systemic adjuvant therapy.