There are almost 10 million new cases of tuberculosis (TB) in a calendar year with around 1.7 million deaths. Worse we now have more than half a million patients of multi drug resistant TB ( MDR TB) ,with 10% of these being extensively drug resistant TB ( XDR TB). The definition of MDR TB is drug resistance of TB bacilli to rifampicin and isoniazid, while XDR TB constitutes added resistance to quinolones and second line injectables like amikacin and capreomycin. Successful treatment of MDR TB is quite difficult with an average success rate of less than 50%. Treatment is of long duration and extends for at least 20 months. Mercifully, WHO has come out with a revised simpler protocol for managing MDR-TB.Medicines have been regrouped based on latest clinical evidence.
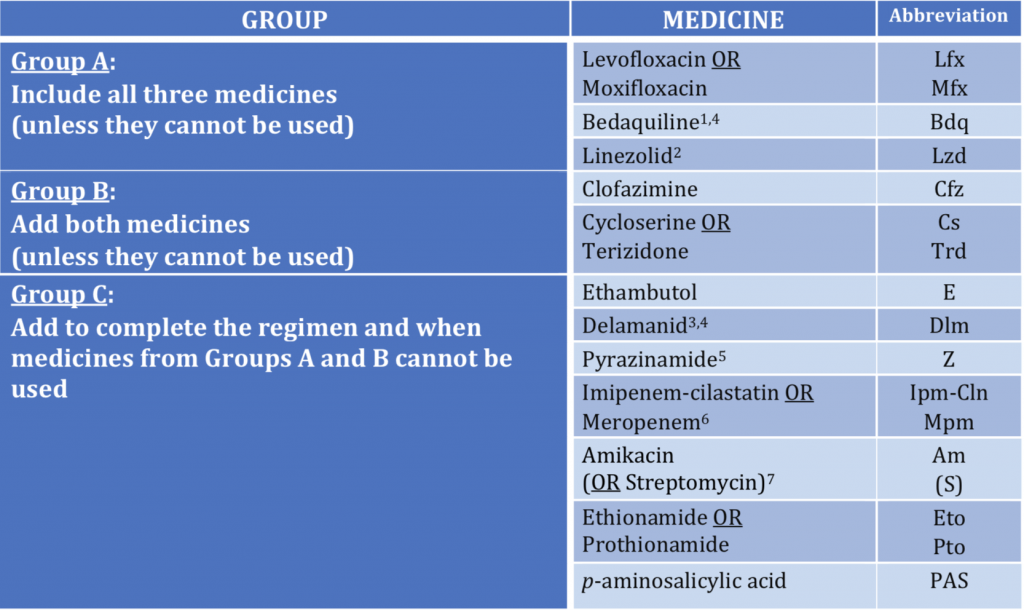
Group A : medicines to be prioritised , Levofloxacin, bedaquiline, linezolid.
Group B: medicines to be added next: cycloserine, terizodone, clofazimine.
Group C: medicines to complete regimen or when drugs from groups A and B cannot be used; ethambutol, pyrazinamide, delamanid, ethionamide, prothionamide, PAS, streptomycin, meropenam, imipenam.
Kanamycin and capreomycin are no longer recommended because of relapse, toxicity and treatment failure.
A shorter regimen lasting 9 to 12 months has been observed to have similar efficacy as with the longer schedule, but with a higher chance of relapse. Quinolone, bedaquiline and linezilod have become top priority drugs while the injectables have been downgraded if not removed entirely from the list. Delamanid comes in group C because of limited data regarding its efficacy.
We now have a double blind randomized trial published with delamanid compared to placebo against optimised background MDR TB treatment in more than 500 MDR TB patients. Delamanid was given as 200 mg once a day for 2 months followed by 100 mg twice a day for the following 6 months. In the last 40 years delamanid is the only new drug discovered for treating MDR TB apart from bedaquiline. The primary endpoint of this study was sputum culture conversion at 6 months.

An earlier smaller randomised study demonstrated significantly greater sputum culture conversion ( SCC) with delamanid as compared to placebo at 2 months ( 45% vs. 30%). Delamanid was however found to increase QT interval. Secondary endpoint was development of resistance to other anti MDR TB drugs over 6 months. Time to SCC was 51 days in the delamanid group versus 57 days in the placebo group, the difference was not significant. Adverse events were similar in both groups while about 4% died in both cohorts.
Although delamanid was found to be effective in the above trial the results are not robust enough for it to be upgrade just yet. More research is needed to better understand safety and efficacy of delamanid in the treatment of MDR TB.
Dying from tuberculosis can be quite unpleasant. The bacilli eats up lung tissue replacing it with blood and liquid waste. The unfortunate victim if not treated literally drowns in himself. Four thousand people die from TB in one single day, TB kills more than malaria and HIV combined. The disease has little respect for borders or visas, it cares little about how wealthy you are. It is estimated that less than 1% of MDR TB patients get optimal treatment. The WHO strives to eradicate TB by 2030, this borders on the ridiculous with the millions of new cases each year and the overwhelming deaths due to TB. Seventeen lakh deaths in a year is a conservative figure by any standards. Yet little is mentioned about this scourge in Indian newspapers or websites. India has the largest pool of TB patients in the world. The reasons for MDR TB are incomplete treatment of index TB; it has also been realised that a fresh patient gets created by transmission of drug resistant bacteria from a known MDR patient. A new MDR patient may need to take 14,600 tablets for 2 long years with no assurance of cure.
The development of rapid diagnostic tests for TB and the new drugs may immensely aid in converting long arduous and toxic treatment into more patient friendly ones. Bedaquiline, another new anti TB drug operates differently ; it acts against ATP synthetase of the TB bacilli and thereby kills it by reducing energy stores. Bedaquline was tested in a randomised manner in 47 patients with MDR TB in double blind fashion. In addition to background anti TB drug regimen bedaquiline or placebo was administered. Sputum conversion was seen in 48% in the bedaquiline group in comparison to only 9% placebo patients. The background treatment consisted of kanamycin , cyclosereine, ofloxacin, pyrazinamide, and ethionamide. I remember this regimen by the pneumonic “C-O-P-E.”
In the second stage bedaquiline was given for 24 weeks (6 months) versus placebo in double blind fashion while background treatment continued for 18 to 24 months. Sputum culture conversion was much earlier with bedaquiloine than with placebo 983 versus 125 days; p<0.01). However there were more deaths in the bedaquiline group than in the placebo group, 2 deaths in the placebo group (81 patients) but 10 deaths occurred in the bed aquiline group (79 patients). Five of 10 deaths were due to disease progression while 1 patient died in a car accident. Four deaths could not be explained, and the FDA was compelled to insert multiple warnings. A strong probability may be QT prolongation by bedaquiline. Despite the fear of increased deaths, bedaquline enjoys group A status because of the ability for sputum conversion in sputum confirmed MDR TB patients.
Bedaquiline’s manufacturer Johnson & Johnson wants to retain the patent, but this would prevent generic forms to be developed. Ironically the patients who shall need it the most may not be able to afford it in India. Currently bed aquiline is priced at 400$ as reported in the Indian press, a reduction of 50% in the price. Bedaquiline will be a boon for MDR TB patients who in the past suffered from irreparable hearing loss by the injectables kanamycin and capreomycin.

The Lancet published a large meta analysis that included 12,000 patients treated for MDR TB ( lancet 2018;392:821-34).The researchers reported a success rate of 61%, failure or relapse of 8%, death rate of 14%; but also that capreomycin and kanamycin resulted in worse clinical outcomes including death. Bedaquiline reduced mortalit, but the meta analysis is limited by data that is observational. The investigators rightly concluded that the results of their meta analysis should motivate others to conduct randomised trials to adequately assess drugs found to be effective in their study; namely linezolid,bedaquiline, later generation fluoroquinolone, clofazimine and carbapenems. They also recommend that capreomycin, kanamycin, ethionomide, protionamide and para aminosalycilic acid be reassessed because of uncertain benefit. The latest WHO recommendations are based to a large extent on this meta analysis.