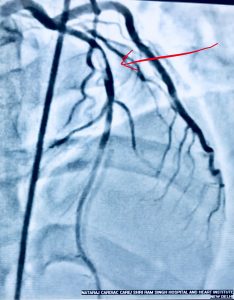
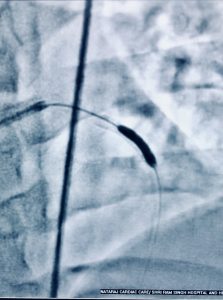
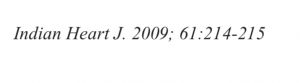Recently a 33 year old man who had undergone primary PCI for acute anterior ST segment elevation myocardial infarction (STEMI) with a bare metal stent inserted in the proximal left anterior descending coronary artery a little more than 8 years ago presented with rest angina of increasing severity in the previous 2 days. The twelve lead ECG showed ST segment coving in precordial leads and his troponin was considerably raised. Coronary angiography revealed in stent restenosis (ISR) accompanied by very late stent thrombosis (VLST). The patient was successfully treated with intracoronary tirofiban bolus followed by intravenous infusion of irofiban alongside balloon angioplasty (BA) with successive larger diameter balloons at high pressure. The patient was discharged the next day in stable condition on dual antiplatelets (that included ticagrelor),a statin, and an ace inhibitor.
Very late stent thrombosis occurs in BMS, albeit at a lower rate than with DES. In stent neoatherosclerosis with ruptured plaque and thin cap fibroatheromas has been observed in BMS. Atheroscrelotic plaques harvested from patients with very late stent thrombosis and those with acute coronary syndrome unrelated to stent thrombosis are histologically indistinguishable from each other. Disruption of neoatherosclerosis inside BMS may be an important underlying mechanism of very late stent thrombosis after 3 years of BMS implantation. The mechanism for VLST in DES on the other hand is largely inflammation at strut site.
Balloon angioplasty was chosen in the patient because of its simplicity and reasonable cost. Balloon angioplasty was one off the earliest strategies used to treat ISR. Lumen enlargement results from stent expansion and tissue extrusion. During the procedure balloon to artery ratio of 1.1 : 1 is selected. Crucially the stenosed part is tackled rather than entire stented segment to prevent dissection or trauma. Some operators use only non compliant balloons.
Evidence on management of ISR however indicate that BA is the least effective technique. A better strategy would have been employment of a drug eluting balloon (DEB) or deployment of a second generation drug eluting stent. The patient concerned was from a poor socioeconomic background and without insurance cover. The fact that he was having an impending anterior STEMI did not give us the luxury of procuring a DEB.
Treatment of ISR continue to be a challenge. Bare metal stents have been used extensively in the past; they have been associated with a relatively high restenosis rates. The main cause of ISR in BMS is neointimal proliferation rich in smooth muscle cells. There is the added spectre of very late stent thrombosis ( VLST) which is defined as stent thrombosis occurring more than one year after the index procedure. Histopathological studies have reported that VLST in a BMS is usually due to neoatherosclerosis accompanied by a thin cap, which is susceptible to rupture and thrombus formation.
Rate of neointimal proliferation were substantially reduced with the drug eluting stent (DES), and hence rates of ISR. The need for reintervention in DES is therefore significantly less than with a BMS, but first generation DES were plagued by late stent thrombosis. Rates of restenosis with DES hover around 10 to 12 %. Treatment of ISR consists of BA (recurrence rates 35% to 60%) , intracoronary irradiation (16% to 23%), and insertion of DES (restenosis 20%). Treatment of ISR in DES by another DES can result in repeat stenosis upto 43%.
There are significant differences between ISR in BMS compared to DES. ISR in DES is more often focal and affect stent edges. But one important reason for ISR both in DES and BMS is an under expanded stent at index procedure. Stent fractures may also initiate focal ISR or stent thrombosis in DES.
Despite no clarity on best treatment of ISR, the evidence is gradually evolving to a strategy of deployment of a second generation DES such as an everolimus eluting stent (EES) or use of a drug eluting balloon (DEB). The jury is still out on whether DES or DEB is better in treating ISR. The latest trial named DARE randomised 278 patients with ISR to treatment with an EES or DEB. The investigators used >50% stenosis as definition of ISR. The DEB used in the trial was a balloon that eluted paclitaxel or PEB. One hundred and thirty seven patients received treatment with a PEB while 141 patients got a EES. Almost 80% patients in both groups underwent 6 month follow up angiography. The study was designed to address non inferiority of PEB vs. EES for the primary endpoint of in segment minimal luminal diameter (MLD) at 6 months.
At 6 months the MLD was similar in both groups. At 12 months death, myocardial infarction and target vessel revascularisation were similar in both groups. Immediate post intervention angiography showed better results with EES but then ate lumen loss was greater with EES, neutralising the gains achieved acutely. Importantly DARE included patients with both DES (55% ISR) and BMS (45% ISR). The results reported by DARE endorse the ISAR- DESIRE 3 trial ( Lancet 2013;381:461-467) that compared PEB with the first generation paclitaxel eluting stent (PES). The ISR-DESIRE 3 trial randomised 402 patients with ISR on DES in a 1:1:1 manner to receive a PEB, PES or ballon angioplasty alone. Follow up angiography at 6-8 months revealed that diameter stenosis were similar with PEB and PES (about 38%), but angiographic results in both were superior to BA that had diameter stenosis of 54%. Frequency of death, myocardial infarction or target lesion thrombosis did no differ between groups. The researchers concluded that by obviating need of additional DES implantation,PEB could be useful in treatment for ISR after implantation of DES.
The RIBS IV trial randomised 309 patients with DES-ISR to DEB or EES. At late angiography ( median 247 days) , patients with EES had significantly greater MLD than patients treated with DEB. Moreover at 12 months follow up clinical events (composite of cardiac death, MI and target vessel vascularisation) was significantly less in the EES arm, mainly driven by fewer rates of target vessel revascularisation. The DEB used in RIBS-IV was the paclitaxel eluting balloon. In patients assigned to DEB, a 1.1:1 balloon to artery ratio was selected using nominal pressures (12 to 14 atm) for 60 secs (JACC 2015;66:23-33).
The RIBS-V trial ( JACC 2014; 63: 1378-1386) randomised 189 patients with BMS-ISR to PEB or EES. At late angiography (292 days) the MLD was significantly more in the EES group as compared to the PEB group. Late loss and restenosis were very low and similar in both groups. Clinical outcomes (death, M I and target vessel revasularisation) , unlike the RIBS-IV with DES-ISR, were similar in the PEB and EES groups. The authors concluded that in patients with BMS-ISR, both PEB and EES provide excellent clinical results with very low rate of clinical and angiographic recurrence.
We now have 2 randomised trials that conclude non inferiority of a DEB as compared to EES in patients with BMS-ISR. The acute luminal diameter gains obtained with EES in the DARE trial was offset by late luminal loss. Both DARE and RIBS-V report similar rates of death, myocardial infarction and target vessel revasularisation with DEB as compared to EES. The ISASR-DESIRE 3 trial had also shown similar clinical results with a PEB as compared to PES. Hence the paclitaxel eluting balloon has shown similar clinical results as compared to a DES and may be an excellent alternative. There is always the advantage of avoiding additional metallic struts and subsequent stent thrombosis when using a DEB. The long term clinical results with DEB in treating ISR need to be confirmed in an adequately powered randomised trial. We also need more data based in intravascular imaging to figure out the mechanisms of ISR. No interventional cardiologist likes the idea of inserting a new stent in an already placed stent. A 3 mm diameter then can become 2.5 mm diameter and could be further reduced in the event of insertion of another stent. A DEB therefore seems to be a better way of treating ISR; at the least it is a viable alternative to a DES with similar clinical outcomes up to one year.